FDA Signs Off on Bivalent COVID Shot as Fourth Dose for Kids Under 5 With Literally No Data
The FDA showed once again that it doesn't care about your kids after it signed off on infants getting fourth doses of the clot shots. Babies could have eight COVID vaccine exposures by age 10 months.
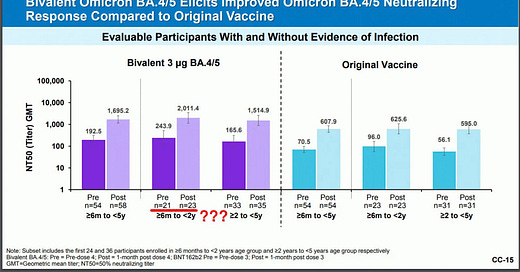
The U.S. Food and Drug Administration (FDA) hit a new low on Tuesday, authorizing a fourth COVID vaccine dose for the nation’s smallest children.
The agency authorized a booster dose of Pfizer’s experimental bivalent COVID-19 vaccine for kids under five years old who previously received the three-dose primary series. Theoretically, an infant could have …