FDA Loses War on Ivermectin, Agrees to Remove Social Media Posts
The FDA agreed to remove and never republish its website and social media posts discouraging people from using ivermectin off-label for the prevention and treatment of COVID-19.
The U.S. Food and Drug Administration (FDA) on March 21 agreed to remove and never republish social media posts and a website article warning against off-label use of ivermectin for the prevention and treatment of COVID-19.
The agreement is part of a settlement in a landmark case filed against the FDA, Department of Health and Human Services (HHS), HHS Secretary Xavier Becerra, and FDA Commissioner Dr. Robert Califf.
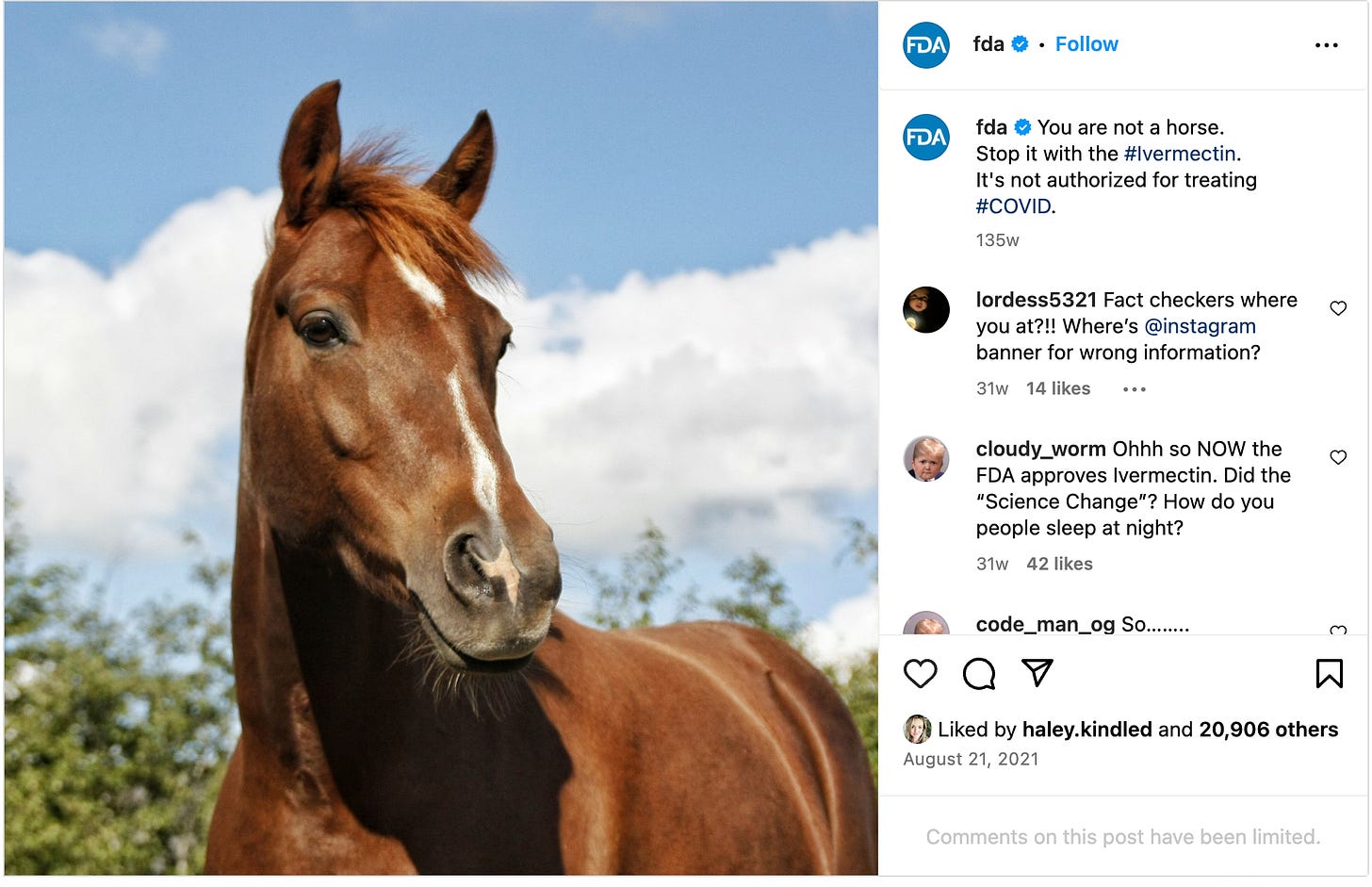
The posts that must be removed include a 2021 post with a picture of a horse that states, “You are not a horse. You are not a cow. Seriously, y’all. Stop it,” and a link to an agency article entitled “Why You” Should Not Take Ivermectin to Treat or Prevent COVID-19.”
A post published on Instagram must also be removed. It read, "You are not a horse. Stop it with the #ivermectin. It's not authorized for treating #COVID."
Additionally, the FDA must remove a Twitter post that states, "Hold your horses, y'all. Ivermectin may be trending, but it isn't authorized or approved to treat COVID-19.
Drs. Mary Bowden, Paul Marik, and Robert Apter, on June 2, 2022, filed a lawsuit in federal court alleging the FDA exceeded its authority as a federal health agency and illegally interfered with the practice of medicine when it aggressively tried to prevent them from using ivermectin to treat COVID-19.
The complaint cites U.S. code stating the FDA “may not interfere with the authority of a health care provider to prescribe or administer any legally marked device to a patient for any condition or disease within a legitimate health care practitioner-patient relationship.”
The lower court initially dismissed the case because the FDA had “sovereign immunity,” which prohibited plaintiffs from suing the federal agency, but the ruling was overturned in September 2023 by the 5th Circuit Court of Appeals and remanded to the lower court.
The Court of Appeals ruled that the FDA identified no authority allowing it to recommend consumers “stop taking medicine" or to otherwise offer medical advice in “tweet-sized doses."
“FDA is not a physician. It has authority to inform, announce, and apprise but not to endorse, denounce, or advise,” wrote Judge Don Willett.
The lower court then ruled only Dr. Bowden had standing to sue the FDA, but before the case could proceed HHS asked the plaintiffs for a settlement.
“We are extremely pleased with the outcome of the settlement as it is a victory for every doctor and patient in the United States,” said Dr. Marik, chairman and chief scientific officer of the FLCCC Alliance and former Chief of Pulmonary and Critical Care Medicine at Eastern Virginia Medical School.
“The FDA interfered in the practice of medicine with their irresponsible language and posts about ivermectin. We will never know how many lives were affected because patients were denied access to a lifesaving treatment because their doctor was ‘just following the FDA,’” he added.
Prior to the settlement agreement, the FDA removed its misleading webpage about ivermectin and COVID-19—although an archived copy is still available.
All of its social media posts are still online. Given the FDA’s track record, it could take the agency 75 years to delete them.
X: @megan_redshaw
Instagram: megan.redshaw
Telegram: @realmeganredshaw






Don't forget the UV ass lights and the bleach, idiots.
How absurd that the FDA is constrained from warning against the inappropriate use of a drug effective against roundworm infestations inappropriately for a viral infection. This is ignorant political encroachment on an area, Clinical Medicine, where politics can only do damage.